Polymyalgia rheumatica (PMR) and giant cell arteritis (GCA) are two related, immune-mediated, inflammatory conditions that occur in the elderly. PMR coexists in 40% of patients with GCA. Similarly, 10% of PMR patients develop GCA at some point during their disease course. The relation between PMR and GCA is further demonstrated by their preference for similar patient populations, linkage to the same HLA haplotypes, similar cytokine patterns in temporal artery biopsies, and similarities in anatomic involvement on PET imaging.1-3 PMR and GCA represent two extremes of a disease spectrum.
Polymyalgia rheumatica
Definition
Bruce is credited with the first description in 1888 of PMR, which he described as “senile rheumatic gout.”4 However, Barber coined the term polymyalgia rheumatica in 1957, and it has become the universally accepted name for this condition.5
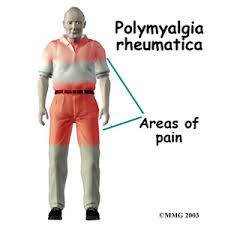
PMR is characterized by proximal, symmetrical musculoskeletal pain and stiffness. Symptoms of systemic inflammation are also common. A dramatic response to low-dose corticosteroids can be a valuable diagnostic tool in patients for whom the diagnosis is uncertain. The lack of response to prednisone raises the possibility of a paraneoplastic process manifesting with proximal pain and stiffness. In patients who have a dramatic response to treatment there is still a need for caution because some patients (~11%) with an initial PMR-like presentation evolve into a phenotype that is more that of rheumatoid arthritis and, less often, other systemic rheumatic illnesses.
6Epidemiology
PMR has a predilection for patients older than 50 years. The mean age at onset is 73 years, and women are affected more often than men. Its annual incidence in Olmstead County, Minnesota, a population with mostly Scandinavian heritage, is 59 per 100,000. The annual incidence of the disease increases with age.7 Whites of northern European descent have a higher incidence of disease than people of African American or Latin American descent.8,9
Pathophysiology
Much has been learned about PMR and GCA, but their cause remains unknown. Their cause is likely multifactorial, resulting in the interplay of age, environment, and genetic susceptibility. The suggestion that PMR may be a forme fruste of GCA was first advanced in the 1950s and 1960s.5 The pathophysiology for both diseases is similar, with abnormalities of cellular immunity leading to vessel and systemic inflammation. Sixteen percent to at least 20% or more of PMR patients demonstrate arteritis on histologic examination, requiring the diagnosis to be changed to GCA.10 Cytokines such as interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α are important in the development of inflammation in GCA.11Messenger RNA (mRNA) for interferon-gamma (IFN-γ) and IFN-γ protein, a product of Th1 lymphocytes, is found in the arterial wall of GCA patients. This suggests that IFN-γ may be a necessary element in the development of vasculitis. The classic histologic features of GCA, which include inflammatory cells involving the adventitia of a muscular artery and migrating toward the media and intima, consist of Th1 cells, dendritic cells, and macrophages.9
Signs and Symptoms
Most patients describe subacute onset of symptoms that remain persistent over time. Seventy percent to 95% of patients report symmetrical shoulder girdle pain and stiffness. Fifty percent to 70% report neck and pelvic girdle pain. Concurrent pain in the upper arms and thighs is common and is usually worse in the morning. Shoulder and leg discomfort can lead to difficulty dressing, hair grooming, and rising from a chair. One third of patients have flulike symptoms described as fever, malaise, anorexia, or weight loss.12
Physical examination findings may reveal pain that limits active range of motion in the shoulders and hips. Passive range of motion should be normal. Despite subjective symptoms of muscle weakness, muscle strength testing should be normal unless it is affected by examination discomfort or by another condition.12 Approximately 50% of patients have been said to present with distal extremity abnormalities including swelling of the knees, wrists, or metacarpophalangeal joints. Other reported findings include soft-tissue swelling; pitting edema of the hands, ankles, and feet; and median nerve compression. However, these findings are typical of inflammatory joint disease and not PMR. The examiner should direct the evaluation along other lines in attempting to define another diagnosis. Frank synovitis of the hands or feet should suggest rheumatoid arthritis or another inflammatory arthropathy. Thus, further laboratory and imaging may be needed to differentiate the two. (See the chapter “Rheumatoid Arthritis”).
Diagnosis
The diagnosis of PMR is based primarily on clinical features. Elevated acute phase reactants provide secondary support for the diagnosis. The erythrocyte sedimentation rate (ESR) is greater than 40 mm/hr in 90% of cases. Other laboratory findings include an elevated C-reactive protein (CRP), normocytic normochromic anemia, thrombocytosis, and elevated alkaline phosphatase. Elevation of muscle enzymes, such as creatine kinase and aldolase, is not a feature of PMR and should prompt consideration of an alternative diagnosis.
In 1979, Bird and colleagues proposed diagnostic criteria for PMR (Table 1). Patients who fulfilled any three criteria or had one criterion along with vasculitis on a temporal artery biopsy were considered to have PMR.13 In 1984, Healy proposed that patients older than 50 years, seronegative for rheumatoid factor, and any three clinical features (neck, shoulder, or pelvic girdle pain, morning stiffness, elevated ESR, or rapid response to low-dose steroids) have PMR.14 Although these criteria should serve as guidelines for the diagnosis of PMR, most authorities agree that no single feature is necessary to diagnose it in all cases. The features noted in these criteria are common enough that if patients present without these symptoms or have a suboptimal response to corticosteroids, the diagnosis should be reconsidered. Conditions that can mimic PMR include malignancies, chronic infections, drug reactions, and other rheumatic conditions such as seronegative rheumatoid arthritis or polymyositis.6
Table 1: Often-Cited Diagnostic Criteria for Polymyalgia Rheumatica
| Authors and Year Proposed | Proposed Criteria | Requirement for Making Diagnosis |
|---|
| Bird et al. (1979) | - Age ≥65 yr
- Bilateral shoulder pain and stiffness
- Acute or subacute onset (<2 wk)
- ESR >40 mm/hr
- Depression and/or weight loss
- Bilateral tenderness in upper arm muscles
- Morning stiffness >1 hr
| Any three of these criteria, or any one plus positive temporal artery biopsy |
| Jones and Hazelman (1981) | - ESR >30 mm/hr or CRP >6 mg/L
- Shoulder and pelvic girdle pain
- Exclusion of rheumatoid arthritis or other inflammatory arthropathy, myopathy, malignancy
- Morning stiffness >1 hr
- Rapid response to corticosteroids
| All criteria must be met |
| Chuang et al. (1982) | - Age ≥50
- ESR >40 mm/hr
- >1 mo bilateral aching and stiffness of at least two of the following areas: Neck or torso, shoulders or proximal arms, hips or proximal thighs
- Exclusion of other causes
| All criteria must be met |
| Healey (1984) | - >1 mo of neck, shoulder, or pelvic girdle pain (any two areas)
- Morning stiffness >1 hr
- Elevated ESR (≤40 mm/hr)
- Exclusion of other diagnoses
- Rapid response to daily, low-dose steroid therapy (e.g., prednisolone ≤20 mg)
| All criteria must be met |
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
© 2004 The Cleveland Clinic Foundation.
Treatment
The first successful use of corticosteroids in patients with PMR was reported by Kersley in 1951.5 Since that time, it has remained the cornerstone of therapy for PMR. Prednisone or prednisolone is the most commonly used corticosteroids. Starting doses range from 15 to 20 mg per day. A dramatic response to therapy with near-total relief of symptoms should occur within 1 to 5 days. Lack of a dramatic response to corticosteroids should prompt physicians to reconsider the diagnosis. A gradual decline of the acute phase reactants should be expected but should never be the sole gauge of therapy. After an adequate response to corticosteroids has been achieved, the initial dose should be maintained for 1 month before beginning a slow taper to the lowest effective dose. One to 2 years of treatment with corticosteroids should be expected, and a few patients require low-dose prednisone for several years.15
Disease flares during the corticosteroid taper are common and often require temporary increases in therapy. Disease flares can occur in the presence of normal acute-phase reactants. Increases in acute phase reactants mandate an evaluation to be sure that comorbid conditions are not responsible for such changes. Isolated increases in acute-phase reactants should lead to more careful monitoring and not to reflexive increases in corticosteroids doses.16
Corticosteroids are the cornerstone of therapy, but they are not without side effects. Most patients have at least one relapse as corticosteroids are tapered, and adverse events occur in almost every patient. The role of immunosuppressive agents other than corticosteroids in PMR is controversial. Methotrexate has been studied in two randomized, double-blind, controlled trials. Van der Veen and colleagues reported that patients randomized to take oral methotrexate (10 mg/week) had the same number of relapses and received the same total cumulative prednisone dose compared with patients who received placebo.17 Caporali and colleagues reported that patients randomized to oral methotrexate (10 mg/week) for 48 weeks had fewer relapses and required lower cumulative prednisone doses than patients who took placebo.18 However, further review of the patients who received methotrexate revealed that they had the same number of relapses while they were taking prednisone as did patients who received placebo. Furthermore, the number of corticosteroid-related adverse events was equal in both treatment groups and the total cumulative prednisone dose reduction achieved by taking methotrexate in place of placebo equaled only about 1 mg/day.19
TNF-α, a cytokine produced by macrophages and T-lymphocytes, appears to play a significant role in the inflammatory process of PMR and GCA. In one pilot study, 3 mg/kg of intravenous infliximab was administered as adjunctive therapy to patients on corticosteroids. This therapy allowed 12 months of remission in three out of four patients treated with the drug.20 These results were not seen in a recent double-blind, randomized, placebo-controlled study by the same author. Patients who received 3 mg/kg of infliximab at the same dosing intervals as used for rheumatoid arthritis over 22 weeks experienced the same number of weeks in remission as those who received placebo. No difference was seen in the duration of corticosteroid therapy or in the total number of patients who were able to discontinue corticosteroids between the groups.21
Outcomes
Adequate treatment with corticosteroids allows most patients to remain symptom free. Patients who have PMR need continued follow-up to monitor for drug-related toxicities and for possible progression to GCA. The development of a new headache or visual changes should prompt immediate medical evaluation and institution of higher doses of corticosteroids. Bilateral upper- and lower-extremity blood pressures should be obtained periodically. Differences between contralateral extremity pressures of 10 mm Hg or more should be considered abnormal. Bruits over carotid, subclavian, or femoral arteries may be due to either atheromatous disease or GCA and require further evaluation by vascular imaging.
Giant cell arteritis
Definition

GCA is a vasculitis characterized by granulomatous inflammation of medium-sized and large arteries. Inflammation is seen more commonly in the extracranial branches of the carotid arteries and other primary branch vessels of the aortic arch. Less often, internal branches of the carotid are affected, most notably the ophthalmic and posterior ciliary arteries; stenosis or occlusion of these arteries can cause loss of vision. At least one out of five patients develop large-vessel inflammation that can lead to branch vessel (e.g., most often subclavian) stenosis and less often aneurysmal dilation of the aorta (especially aortic root) or branch vessels.
22 GCA is the most common vasculitis in whites older than 50 years. It has a predilection for people of northern European heritage. Women are affected at least twice as often as men.
9Prevalence
In the United States, GCA affects about 18 of 100,000 people older than 50 years. The epidemiologic characteristics of GCA are similar to those of PMR. The incidence of GCA is much higher in the northern latitudes, with a mean age at onset of 74 years. GCA and PMR might represent opposite ends of a disease spectrum, with many patients presenting features of both diseases. Approximately 40% of GCA patients have concurrent features of PMR at some point during their disease course.23
Pathophysiology

Figure 1: Click to Enlarge
The cause of GCA is unknown, but vessel inflammation is cell mediated and not autoantibody induced. Dendritic cells, macrophages, and Th1-lymphocytes enter the vessel wall via the vasa vasorum and spread through the arterial adventitia.24 A small fraction of activated T lymphocytes in the artery wall become clonally expanded. The cause for the clonal expansion is unknown but may be from a yet-unidentified neoantigen present in the arterial wall.25 A broad range of proinflammatory cytokines, growth factors, and metalloproteinases are associated with inflammatory cell migration throughout the arterial media and intima. This panarterial inflammation leads to arterial damage, intimal proliferation, and ultimately luminal narrowing (Fig. 1). The luminal narrowing is responsible for ischemic events (loss of vision, stroke, and claudication). Understanding how these cytokines participate in inflammation will lead to better-targeted therapies in both GCA and PMR.26
Signs and Symptoms
Headache is the most common symptom in GCA and occurs in 63% to 87% of patients. Systemic symptoms including fever, weight loss, and myalgias occur in 50% of patients.27 Other symptoms include scalp pain, jaw pain while chewing, and arm or leg claudication. Vision loss, the most dreaded complication of GCA, occurs in more than 30% of patients.28Anterior ischemic optic neuropathy is the most common cause of blindness. Twenty-seven percent of patients develop either an aortic aneurysm or large artery stenosis at some point during their disease course. Six percent of patients who develop an aortic aneurysm present with symptoms consistent with a dissection of the aneurysm.22 Extremity claudication occurs when aortic branch vessels, such as the subclavian artery, become critically narrow. Stroke and vision loss can occur without any preceding symptoms; however, patients can present with insidious nonspecific symptoms before the diagnosis of GCA is made. Forty percent of patients present with symptoms not considered classic for GCA. These symptoms can include cough, throat pain, or tongue pain.
A thorough physical examination may reveal a prominent, tender temporal artery. Evaluation of the artery may reveal a decreased pulse and a nodular appearance. Asymmetrical extremity blood pressures or pulses, bruits over subclavian or carotid arteries, or a murmur of aortic insufficiency suggests aortic or primary aortic branch involvement.
Diagnosis
Similar to PMR, no serologic test is diagnostic for GCA. Diagnosis is based on clinical symptoms in the presence of abnormal acute-phase reactants. More than 90% of patients have an elevated ESR. An elevated CRP, alkaline phosphatase, and platelets are not uncommon. Temporal artery biopsy is considered the standard diagnostic test for GCA. The sensitivity of biopsy, in series from medical practitioners, in detecting GCA is about 50%.29 The yield of biopsy is a function of pretest probability, which might explain why some ophthalmology and other series, in which visual abnormalities were common, have yields as high as 80%.30 Biopsy of the contralateral temporal artery adds very little to the sensitivity of the test.31

Figure 2: Click to Enlarge
Imaging of the vessel lumen with arteriography or magnetic resonance arteriography (MRA) may reveal aortic or arterial branches with stenoses or aneurysms (Fig. 2). The subclavian arteries, carotid arteries, and ascending aorta are the most commonly affected areas. Other arterial branches, such as the mesenteric and renal arteries, can also be affected. Vascular PET imaging with 18F-fluorodeoxyglucose may reveal vessel uptake in GCA as well as PMR. It is a more sensitive marker for disease than biopsy, but its diagnostic specificity is still in the process of being defined.32
Treatment
Corticosteroids are the drug of choice for the treatment of GCA. They quickly reduce symptoms and decrease risk of visual complications from 60% to 14%.33 Therapy with corticosteroids should start when GCA is first suspected. Waiting to start corticosteroids until after a temporal artery biopsy could result in irreversible loss of vision.
The optimal initial dose of prednisone is unclear, but most authorities agree that the initial dose should be between 40 and 60 mg/day. Doses of at least 60 mg are preferred when presenting features include ophthalmic or neurologic complications. Some authorities advocate intravenous methylprednisolone at doses of 1000 mg a day for 3 to 5 days in patients presenting with blindness.33-35 The initial oral dose of corticosteroids should continue for 1 month before taper is considered. Many tapering schedules exist but few have been studied in clinical trials. One general rule is to taper by 10% to 20% every 2 weeks.9 Treatment duration is different for each patient. Long-term therapy is required in most patients. It is not unusual for corticosteroid therapy to extend beyond 4 years.36-38
The efficacy of methotrexate in Wegener's granulomatosis and Takayasu's arteritis has led to its use in three randomized, double-blind, placebo-controlled trials for GCA. Jover and colleagues reported that patients randomized to methotrexate doses of 10 mg/week had fewer relapses and lower cumulative steroid doses compared with those who received placebo. However, a difference in relapse rates was only noted after 1 year of disease. There was no advantage to methotrexate in the first year. In addition, patients who received methotrexate had the same number of steroid-related side effects as those who received placebo.39 Hoffman and colleagues conducted the only multicenter, randomized, double-blind, placebo-controlled trial of methotrexate at doses of 15 mg each week. Their conclusions did not support the use of methotrexate as adjunctive therapy with corticosteroids. Patients who were randomized to receive methotrexate did not have reduced disease activity, cumulative corticosteroid doses, or corticosteroid-related toxicities.40 Spiera and colleagues reported similar results.41 The use of adjunctive methotrexate as a steroid-sparing agent in patients with GCA remains a controversial issue.

Figure 3: Click to Enlarge
The finding of abundant TNF-α in GCA arteries (Fig. 3) has led investigators to study TNF-α inhibition as a potential disease modulator in GCA. Hoffman and colleagues reported results of a multicenter, randomized, double-blind, placebo-controlled trial of intravenous infliximab as adjunctive therapy to corticosteroids in patients with newly diagnosed GCA.42The study was stopped at 22 weeks when infliximab was shown to not improve durability of remission or reduce cumulative steroid doses. The results of this study suggest that although TNF is found in abundance in affected vessels, it might not play a critical role in the pathogenesis of GCA. Other mediators might play more important roles in disease propagation.
Aspirin (ASA) is known to reduce the risks of ischemic stroke and myocardial infarction.43,44 No prospective trial has ever been done to see if antiplatelet therapy reduces the risk of cranial ischemic complications in patients with GCA. A recent review of 175 GCA patients by Nesher and colleagues observed that patients who took low-dose ASA (100 mg/day) were five times less likely to present with visual complications or stroke.45 A second report by Lee and colleagues showed similar results in patients on antiplatelet or anticoagulant therapy.46 Sixteen percent of GCA patients taking ASA, warfarin, or clopidogrel developed an ischemic event compared with 48% of patients not on this therapy (P < 0.0005). Bleeding complications were not increased in the patients on antiplatelet or anticoagulant therapy. The authors concluded that adjunctive low-dose ASA should be considered in all patients with GCA who have no contraindications for its use.
Outcomes
Some studies have found that the overall mortality in patients with GCA is similar to that of age- and gender-matched controls. Other studies have noted an increase in mortality, particularly from cardiovascular events. There is agreement that patients with GCA are at higher risk of death from the complications of aortic aneurysms. Thoracic aneurysms are 17 times more likely to occur in GCA patients and can occur at any time during the disease course. Fifty percent of patients with aortic aneurysms experience dissection or rupture of their aneurysm.22,47-49 Cost-benefit data are not available. Because the risks of aortic catastrophes are well documented, we recommend careful auscultation for aortic valve murmurs or bruits, which should be followed by MRA or CT angiography to determine the nature and seriousness of the aortic lesion. Size of the lesion, hemodynamic consequences, and change over time per sequential imaging determine the need for surgical interventions.
Nearly every patient treated with long-term corticosteroids develops complications related to therapy. Sixty percent develop severe adverse events such as corticosteroid-induced diabetes, avascular necrosis, glaucoma, or vertebral fractures.50Corticosteroid-induced osteoporosis is a well-known complication of long-term steroid use. Medications such as calcium, vitamin D, and anti-resorptive agents should be considered in every patient who will receive corticosteroids for more than 3 months. Screening for osteoporosis with bone densitometry at the induction of corticosteroid therapy (and at regular intervals) is as important an intervention as prescribing steroids to prevent blindness.
Summary
- Clinicians should inquire about headache, jaw pain, and vision loss in all PMR patients at every clinic visit because 10% will develop GCA at some point during their disease course.
- Lack of a response to corticosteroids or the inability to taper below 20 mg/day should raise the possibility of a paraneoplastic process in patients with GCA.
- Isolated increases in acute phase reactants in GCA and PMR should never lead to reflexive increases in corticosteroids doses but lead to more careful monitoring for a disease flare.
- Aortic aneurysms occur in about one of five patients with GCA and should be periodically screened for in every patient.
- Corticosteroids remain the standard of care for the treatment of GCA and PMR.
References
- Wagner AD, Goronzy JJ, Weyand CM. Functional profile of tissue-infiltrating and circulating CD68+ cells in giant cell arteritis. Evidence for two components of the disease. J Clin Invest. 1994, 94: 1134-1140.
- Weyand CM, Hicok KC, Hunder GG, Goronzy JJ. Tissue cytokine patterns in patients with polymyalgia rheumatica and giant cell arteritis. Ann Intern Med. 1994, 121: 484-491.
- Blockmans D, Maes A, Stroobants S, et al: New arguments for a vasculitic nature of polymyalgia rheumatica using positron emission tomography. Rheumatology (Oxford). 1999, 38: 444-447.
- Bruce W. Senile rheumatic gout. BMJ (Clin Res Ed). 1882, 2: 811-813.
- Hunder GG. The early history of giant cell arteritis and polymyalgia rheumatica: First descriptions to 1970. Mayo Clin Proc. 2006, 81: 1071-1083.
- Gonzalez-Gay MA, Garcia-Porrua C, Salvarani C, et al: The spectrum of conditions mimicking polymyalgia rheumatica in northwestern Spain. J Rheumatol. 2000, 27: 2179-2184.
- Doran MF, Crowson CS, O’Fallon WM, et al: Trends in the incidence of polymyalgia rheumatica over a 30 year period in Olmsted County, Minnesota, USA. J Rheumatol. 2002, 29: 1694-1697.
- Smith CA, Fidler WJ, Pinals RS. The epidemiology of giant cell arteritis. Report of a ten-year study in Shelby county, Tennessee. Arthritis Rheum. 1983, 26: 1214-1219.
- Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. Ann Intern Med. 2003, 139: 505-515.
- Salvarani C, Cantini F, Boiardi L, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. N Engl J Med. 2002, 347: 261-271.
- Hernandez-Rodriguez J, Segarra M, Vilardell C, et al: Tissue production of pro-inflammatory cytokines (IL-1β, TNFα and IL-6) correlates with the intensity of the systemic inflammatory response and with corticosteroid requirements in giant-cell arteritis. Rheumatology (Oxford). 2004, 43: 294-301.
- Mandell B. Polymyalgia rheumatica: Clinical presentation is key to diagnosis and treatment. Cleve Clin J Med. 2004, 71: 489-495.
- Bird HA, Esselinckx W, Dixon AS, et al: An evaluation of criteria for polymyalgia rheumatica. Ann Rheum Dis. 1979, 38: 434-439.
- Healey LA. Polymyalgia rheumatica and the American Rheumatism Association criteria for rheumatoid arthritis. Arthritis Rheum. 1983, 26: 1417-1418.
- Salvarani C, Cantini F, Boiardi L, Hunder GG. Polymyalgia rheumatica. Best Pract Res Clin Rheumatol. 2004, 18: 705-722.
- Weyand CM, Fulbright JW, Evans JM, et al: Corticosteroid requirements in polymyalgia rheumatica. Arch Intern Med. 1999, 159: 577-584.
- van der Veen MJ, Dinant HJ, van Booma-Frankfort C, et al: Can methotrexate be used as a steroid sparing agent in the treatment of polymyalgia rheumatica and giant cell arteritis?. Ann Rheum Dis. 1996, 55: 218-223.
- Caporali R, Cimmino MA, Ferraccioli G, et al: Prednisone plus methotrexate for polymyalgia rheumatica: A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2004, 141: 493-500.
- Stone JH. Methotrexate in polymyalgia rheumatica: Kernel of truth or curse of tantalus?. Ann Intern Med. 2004, 141: 568-569.
- Salvarani C, Cantini F, Niccoli L, et al: Treatment of refractory polymyalgia rheumatica with infliximab: A pilot study. J Rheumatol. 2003, 30: 760-763.
- Salvarani C, Manzini C, Paolazzi G, et al: Infliximab in the treatment of polymyalgia rheumatica: A double blind randomized, placebo controlled study. Arthritis and Rheumatism. 2005, 52: s676-s677.
- Nuenninghoff DM, Hunder GG, Christianson TJ, et al: Mortality of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis: A population-based study over 50 years. Arthritis Rheum. 2003, 48: 3532-3537.
- Hunder GG, Valente RM. Giant cell arteritis: Clinical aspects. In: Hoffman GS, Weyand CM (eds): Inflammatory Diseases of Blood Vessels. vol 1. New York: Marcel Dekker, 2002, pp 425-441.
- Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med. 2003, 349: 160-169.
- Martinez-Taboada V, Hunder NN, Hunder GG, et al: Recognition of tissue residing antigen by T cells in vasculitic lesions of giant cell arteritis. J Mol Med. 1996, 74: 695-703.
- Weyand CM, Goronzy JJ. Pathogenic mechanisms in giant cell arteritis. Cleve Clin J Med. 2002, 69: (Suppl 2): SII28-32.
- Calvo-Romero JM. Giant cell arteritis. Postgrad Med J. 2003, 79: 511-515.
- Birkhead NC, Wagener Hp, Shick RM. Treatment of temporal arteritis with adrenal corticosteroids: Results in fifty-five cases in which lesion was proved at biopsy. J Am Med Assoc. 1957, 163: 821-827.
- Hoffman GS. Treatment of giant-cell arteritis: Where we have been and why we must move on. Cleve Clin J Med. 2002, 69: (Suppl 2): SII117-20.
- Allison MC, Gallagher PJ. Temporal artery biopsy and corticosteroid treatment. Ann Rheum Dis. 1984, 43: 416-417.
- Younge BR, Cook BE Jr, Bartley GB, et al: Initiation of glucocorticoid therapy: Before or after temporal artery biopsy?. Mayo Clin Proc. 2004, 79: 483-491.
- Blockmans D, de Ceuninck L, Vanderschueren S, et al: Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: A prospective study of 35 patients. Arthritis Rheum. 2006, 55: 131-137.
- Aiello PD, Trautmann JC, McPhee TJ, et al: Visual prognosis in giant cell arteritis. Ophthalmology. 1993, 100: 550-555.
- Chan CC, O'Day J. Oral and intravenous steroids in giant cell arteritis. Clin Experiment Ophthalmol. 2003, 31: 179-182.
- Su GW, Foroozan R. Update on giant cell arteritis. Curr Opin Ophthalmol. 2003, 14: 332-338.
- Wilke WS, Hoffman GS. Treatment of corticosteroid-resistant giant cell arteritis. Rheum Dis Clin North Am. 1995, 21: 59-71.
- Hunder GG, Sheps SG, Allen GL, Joyce JW. Daily and alternate-day corticosteroid regimens in treatment of giant cell arteritis: Comparison in a prospective study. Ann Intern Med. 1975, 82: 613-618.
- Andersson R, Malmvall BE, Bengtsson BA. Long-term corticosteroid treatment in giant cell arteritis. Acta Med Scand. 1986, 220: 465-469.
- Jover JA, Hernandez-Garcia C, Morado IC, et al: Combined treatment of giant-cell arteritis with methotrexate and prednisone. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001, 134: 106-114.
- Hoffman GS, Cid MC, Hellmann DB, et al: A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum. 2002, 46: 1309-1318.
- Spiera RF, Mitnick HJ, Kupersmith M, et al: A prospective, double-blind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA). Clin Exp Rheumatol. 2001, 19: 495-501.
- Hoffman GS, Cid MC, Weyand CM, et al: Phase II study of the safety and efficacy of infliximab in giant cell arteritis (GCA): 22 Week interim analysis. Arthritis Rheum. 2005, 52: S271.
- Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing physicians’ health study. N Engl J Med. 1989, 321: 129-135.
- Ridker PM, Cook NR, Lee IM, et al: A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005, 352: 1293-1304.
- Nesher G, Berkun Y, Mates M, et al: Risk factors for cranial ischemic complications in giant cell arteritis. Medicine (Baltimore). 2004, 83: 114-122.
- Lee MS, Smith SD, Galor A, Hoffman GS. Antiplatelet and anticoagulant therapy in patients with giant cell arteritis. Arthritis Rheum. 2006, 54: 3306-3309.
- Uddhammar A, Eriksson AL, Nystrom L, et al: Increased mortality due to cardiovascular disease in patients with giant cell arteritis in northern Sweden. J Rheumatol. 2002, 29: 737-742.
- Evans J, Hunder GG. The implications of recognizing large-vessel involvement in elderly patients with giant cell arteritis. Curr Opin Rheumatol. 1997, 9: 37-40.
- Evans JM, O’Fallon WM, Hunder GG. Increased incidence of aortic aneurysm and dissection in giant cell (temporal) arteritis. A population-based study. Ann Intern Med. 1995, 122: 502-507.
- Nesher G, Sonnenblick M. Steroid-sparing medications in temporal arteritis—report of three cases and review of 174 reported patients. Clin Rheumatol. 1994, 13: 289-292.
http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/rheumatology/polymyalgia-rheumatica-and-giant-cell-arteritis/
 Your big toe, Hallux or great toe is a very important structure of the proper function of the lower extremity. Most of the people they don’t pay any attention to their toe unless it’s too ugly or hairy or too long and of course when it’s injured.
Your big toe, Hallux or great toe is a very important structure of the proper function of the lower extremity. Most of the people they don’t pay any attention to their toe unless it’s too ugly or hairy or too long and of course when it’s injured. Hallux Limitus can be caused from various circumstances. In athletes, trauma is the most common reason. The degree of trauma can vary from minor repetitive stress to severe trauma. Over pronation is a common cause of repetitive stress that is not felt until is too late.
Hallux Limitus can be caused from various circumstances. In athletes, trauma is the most common reason. The degree of trauma can vary from minor repetitive stress to severe trauma. Over pronation is a common cause of repetitive stress that is not felt until is too late. Under normal conditions, with each step, your foot must expand to absorb and distribute with loading of your bodyweight. Your arch or more specifically, your longitudinal arch takes the brunt of these forces. Your foot and arch then lengthens at which time, your big toe has to extend to tighten up the tendons and ligaments and plantar fascia of your foot to both support these forces and utilize potential energy so you have a little ‘’spring’’ in your step… literally. This is often referred to as the ‘’windlass’’ effect.
Under normal conditions, with each step, your foot must expand to absorb and distribute with loading of your bodyweight. Your arch or more specifically, your longitudinal arch takes the brunt of these forces. Your foot and arch then lengthens at which time, your big toe has to extend to tighten up the tendons and ligaments and plantar fascia of your foot to both support these forces and utilize potential energy so you have a little ‘’spring’’ in your step… literally. This is often referred to as the ‘’windlass’’ effect. No matter what the cause of Hallux Limitus is , repetitive hyperextension and/or compression to the joints of the big toe will eventually cause inflammation resulting in early breakdown of the cartilage protecting the ends of the two bones. Without treatment the degenerative process will continue, forming cartilaginous spurs. With progression these cartilage structures can calcify into bony spurs. The end-stage of this disorder is a condition known as Hallux Rigidus, where the joint literallu fuse and no motion is left within it. Even with this as a possibility, much of the dysfunction that results from hallux limitus is not due to the injury to the joints of the big toe, but the compensation that takes place elsewhere in the body.
No matter what the cause of Hallux Limitus is , repetitive hyperextension and/or compression to the joints of the big toe will eventually cause inflammation resulting in early breakdown of the cartilage protecting the ends of the two bones. Without treatment the degenerative process will continue, forming cartilaginous spurs. With progression these cartilage structures can calcify into bony spurs. The end-stage of this disorder is a condition known as Hallux Rigidus, where the joint literallu fuse and no motion is left within it. Even with this as a possibility, much of the dysfunction that results from hallux limitus is not due to the injury to the joints of the big toe, but the compensation that takes place elsewhere in the body.



![[Thoracic Outlet Image]](http://www.nismat.org/ptcor/thoracic_outlet/thoracic_outlet.gif)
![[Hands-Up Test Image 1]](http://www.nismat.org/ptcor/thoracic_outlet/handsup1.jpg)
![[Hands-Up Test Image 2]](http://www.nismat.org/ptcor/thoracic_outlet/handsup2.jpg)
![[Adson Maneuver Image]](http://www.nismat.org/ptcor/thoracic_outlet/adson.jpg)
![[Costoclavicular Maneuver Image]](http://www.nismat.org/ptcor/thoracic_outlet/costo_man.jpg)
![[Allen Test Image]](http://www.nismat.org/ptcor/thoracic_outlet/allen.jpg)
![[Provocative Elevation Image 1]](http://www.nismat.org/ptcor/thoracic_outlet/prov_elev1.jpg)
![[Provocative Elevation Image 2]](http://www.nismat.org/ptcor/thoracic_outlet/prov_elev2.jpg)
![[Neck (side) Stretch Image 1]](http://www.nismat.org/ptcor/thoracic_outlet/neck1.jpg)
![[Neck (side) Stretch Image 2]](http://www.nismat.org/ptcor/thoracic_outlet/neck2.jpg)
![[Chest Stretch Image 1]](http://www.nismat.org/ptcor/thoracic_outlet/chest1.jpg)
![[Chest Stretch Image 2]](http://www.nismat.org/ptcor/thoracic_outlet/chest2.jpg)
![[Chest Stretch Image 3]](http://www.nismat.org/ptcor/thoracic_outlet/chest3.jpg)
![[Neck Stretch (side) Image]](http://www.nismat.org/ptcor/thoracic_outlet/neck3.jpg)
![[Shoulder-Chest Stretch Image 1]](http://www.nismat.org/ptcor/thoracic_outlet/sh-chest1.jpg)
![[Shoulder-Chest Stretch Image 2]](http://www.nismat.org/ptcor/thoracic_outlet/sh-chest2.jpg)
![[Shoulder-Chest Stretch Image 3]](http://www.nismat.org/ptcor/thoracic_outlet/sh-chest3.jpg)
![[Rib Mobilization Image 1]](http://www.nismat.org/ptcor/thoracic_outlet/rib1.jpg)
![[Rib Mobilization Image 2]](http://www.nismat.org/ptcor/thoracic_outlet/rib2.jpg)







